Crystals ... diamond structure, and something called K_4
By joe
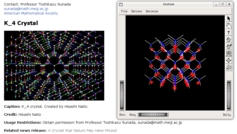
- 1 minutes read - 155 wordsI am going to have to look up the article referred to by /. This morning, they linked to an article in AMS about crystal symmetry, and a structure they called K_4. This structure, they claimed, does not occur in nature. Odd I thought … as the picture they showed, well, I thought I had seen it before.
So using Inventor, I pulled out an old copy of a Gallium Arsenide lattice I used for simulations, more than a decade ago (aren’t open formats nice?) and put it up on the screen, right next to the structure that doesn’t exist in nature.This is what I saw
[
](/images/K_4-vs-GaAs.png)
Not seen in nature? You be the judge. As noted, I need to read the article, it is possible that their picture is mislabeled, and they are showing a diamond lattice where they mean to be showing a different lattice. That said, lattices are extremely well studied.